Signs of the Times: New Male Birth Control To Hit The Market Soon
Warning: Sensitive Material
Doctors are on the cusp of launching the first new male contraceptive
in more than a century. But rather than a Big Pharma lab, the
breakthrough is emerging from a university startup in the heart of rural
India.Years of human trials on the injectable, sperm-zapping product are coming to an end, and researchers are preparing to submit it for regulatory approval. Results so far show it’s safe, effective and easy to use—but gaining little traction with drugmakers. That’s frustrating its inventor, who says his technique could play a crucial role in condom-averse populations.
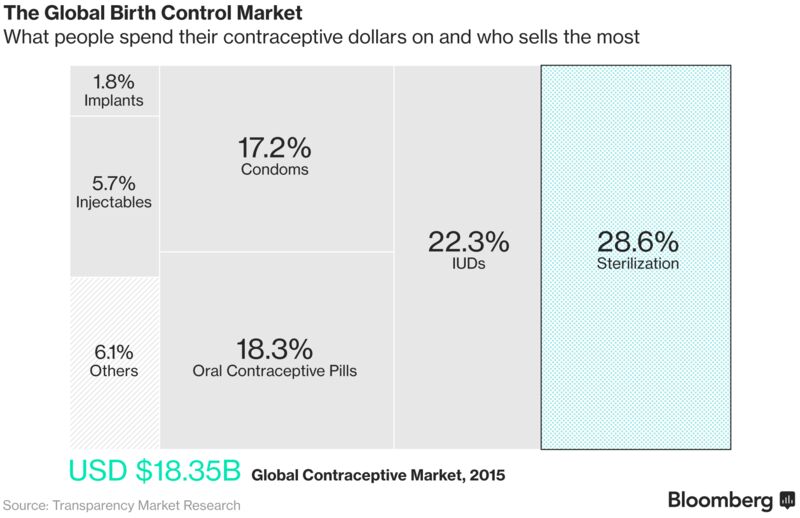
A new birth control method for men has the potential to win as much as half the $10 billion market for female contraceptives worldwide and cut into the $3.2 billion of annual condom sales, businesses dominated by pharmaceutical giants Bayer AG, Pfizer Inc. and Merck & Co., according to estimates from the last major drug company to explore the area. India’s reversible procedure could cost as little as $10 in poor countries, and may provide males with years-long fertility control, overcoming compliance problems and avoiding ongoing costs associated with condoms and the female birth-control pill, which is usually taken daily.
It could also ease the burden on the 225 million women in developing countries, who the World Health Organization says have an unmet need for contraception. Yet, so far only a U.S. non-profit has taken up development of the technology abroad.

Professor Sujoy Guha.
Photographer: Sumit Dayal/Bloomberg
“The fact that the big companies are run by white, middle-aged males who have the same feeling—that they would never do it—plays a major role,” said Herjan Coelingh Bennink, a gynecology professor who helped develop the contraceptives Implanon and Cerazette as head of research and development in women’s health for Organon International from 1987 to 2000. “If those companies were run by women, it would be totally different.”
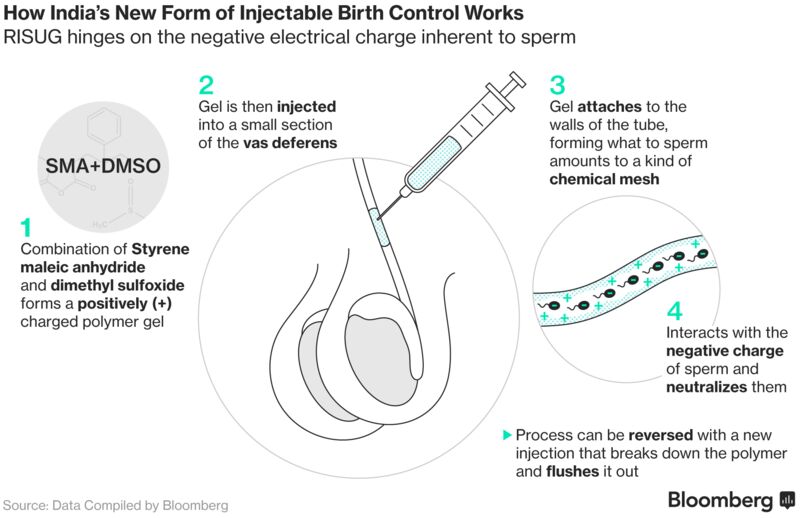
Guha’s technique for impairing male fertility relies on a polymer gel that’s injected into the sperm-carrying tubes in the scrotum. The gel, which has the consistency of melted chocolate, carries a positive charge that acts as a buffer on negatively charged sperm, damaging their heads and tails, and rendering them infertile.
The treatment, known as reversible inhibition of sperm under guidance, or RISUG, is reversed with a second shot that breaks down the gel, allowing sperm to reach the penis normally.
The expected launch of RISUG over the next two years will contribute to the Indian contraceptive market's 17 percent growth through 2021, according to a report last year from Pharmaion Consultants, based near New Delhi.

Professor Guha's assistant examines sperm samples.
Photographer: Sumit Dayal/Bloomberg
A submission to regulators this year will seek approval for RISUG as a permanent method of birth control. That will be appended with clinical data supporting reversibility, Sharma said. India has more married women with an unmet need for family planning than any other country, and social stigma and a lack of privacy in stores has kept condom use to less than 6 percent.
Read More: Durex Wants to Break India's Condom-Buying Taboo
Globally, men tend to take a back seat in matters of contraception. Almost 60 percent of women in spousal relationships used the contraceptive pill or some other form of modern contraception worldwide in 2015, according to a United Nations report. In contrast, 8 percent relied on their male partner using a condom.
Still, there were questions at Organon about whether it would be worthwhile financially to develop a new entrant in the low-margin contraceptives market, and the project was eventually shelved, he said.

A
researcher prepares syringes at the reversible inhibition of sperm
under guidance (RISUG) male contraceptive R&D laboratory.
Photographer: Sumit Dayal/Bloomberg
Bayer, which bought Schering in 2006, stopped all research and development activities around male fertility control about a decade ago, said Astrid Kranz, a company spokeswoman.
Although an earlier clinical trial involving the administration of hormones via injection and an implant was “efficient, with a tolerable side effect profile,” Kranz said, the Leverkusen, Germany-based drugmaker wasn’t convinced this “inconvenient” regimen would find sufficient market acceptance.
Male contraception isn’t an area of active research for Pfizer and Merck either, representatives said. Both companies sell products for female fertility control.
Side effects aside, it would take about $100 million and 10 years to bring a hormone-based male birth control pill to market—a low-priority undertaking for pharmaceutical executives, Coelingh Bennink said.
That’s now the dilemma Indian inventor Guha faces.
“In doing anything abroad, quite substantial money is required, and that can only come from the pharmaceutical industry,” Guha said, surrounded by dusty stacks of paper, books and prototype inventions that bury every surface in his office at the Indian Institute of Technology in Kharagpur, about 130 kilometers (80 miles) west of Kolkata.
In the face of disinterest from the pharmaceutical industry, Guha licensed the technology to the Parsemus Foundation, a U.S.-based non-profit, to help establish a market for it outside India, he said.
The foundation, based in Berkeley, California, is seeking donations to fund costly human trials starting next year after a study in 16 rhesus monkeys published last month showed Vasalgel was successful in preventing conception while the primates fraternized with females for 5 to 24 months.
Guha meantime has registered a startup in India called IcubedG Ideas Pvt. Ltd. through which he is pushing ahead with introducing the technology in his home country. He leased space in a New Delhi industrial zone in January after developing a method of mass production using a government grant. Three couples who participated in the clinical trials gathered in his Kharagpur office in February to attest to the need.

Guha's R&D laboratory located inside the IIT Kharagpur campus.
Photographer: Sumit Dayal/Bloomberg
When a public health worker told the couple about Guha’s promising alternative, Ari decided to enroll in the study. The injection took 15 minutes with some local anesthesia, and after half an hour of observation at the clinic, he said he was able to walk the 2.5 kilometers home. Two days later, he was back at work. Ari was so enthused by the procedure, he convinced two other couples to have it done, he said.
Stories like that encourage Guha to persist with the project, he said, even though patents on his invention have long since expired and he won’t see any personal financial gain even if it takes off worldwide.
“Why should the burden be borne by the female only?” he said in his office after the three couples had left. “There has to be an equal partnership.”
